How neurotransmitters work together to detect and distinguish odors

A longstanding hypothesis in neurobiology was that a single neuron releases a single type of neurotransmitter, a molecule used by neurons to communicate with one another. In recent decades, several neurons have been found to release more than one neurotransmitter. This phenomenon called co-transmission is increasingly gaining recognition as a powerful and versatile molecular mechanism useful for the dynamic regulation of diverse neural circuits. However, precisely how co-transmission affects the firing of target neurons, and the overall behavior of an animal, remains to be elucidated.
A recent study in the laboratory of Dr. Benjamin Arenkiel, professor at Baylor College of Medicine and a principal investigator at the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) has dissected how co-transmission of two neurotransmitters – gamma-aminobutyric acid (GABA) and dopamine – from a specific group of olfactory bulb neurons modulates the activity of the entire circuit. It was published in Cell Reports.
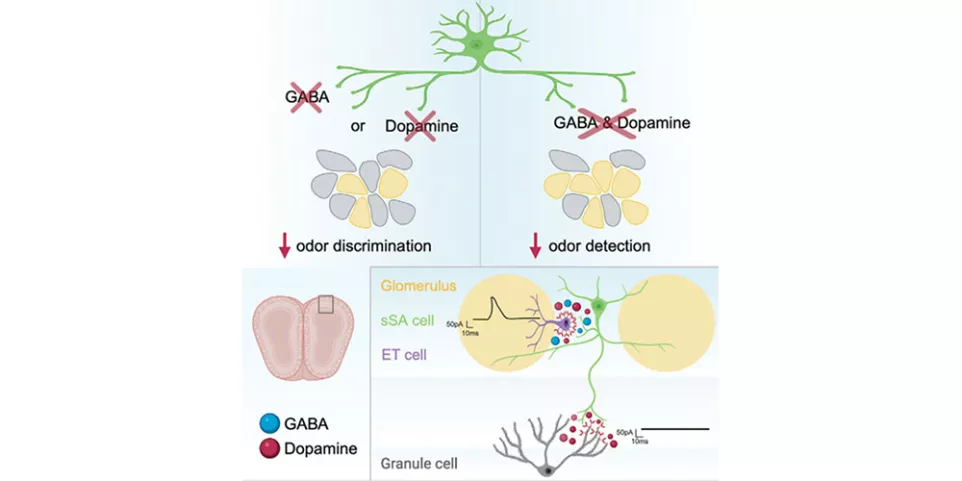
GABA and dopamine are both present and co-transmitted in many brain cells. The Arenkiel lab focused on a type of olfactory inhibitory neuron called the superficial short axon cells (sSACs) that receive inputs from various olfactory sensory neurons.
“To understand the role of sSACs in detecting and discriminating odors, we specifically blocked the release of GABA or dopamine from these neurons,” lead author Dr. Ariel Lyons-Warren, a clinical scientist in the Arenkiel lab, said. “We used a well-established genetic technique to generate mouse models that could not release either GABA or dopamine or both.
Using a behavioral assay that hinges on the innate ability of mice to detect hidden food, they found that mice that lack both GABA and dopamine were unable to detect odors. Inhibiting either of these neurotransmitters individually did not lead to any impairments in odor detection. However, mice in which either GABA or dopamine release was blocked could not differentiate between pairs of molecularly similar odors that normal mice could easily tell apart.
“Based on these observations, we conclude that both GABA and dopamine are individually sufficient to detect odors whereas they likely act cooperatively with one another to discriminate similar odors,” added Dr. Lyons-Warren.
The sSACs receive inputs from various olfactory sensory neurons and send those signals to tufted cells and mitral cells, the primary output neurons that synapse with granule cell neurons present in the deepest layer of the olfactory bulb. Thus, sSACs are a part of the initial circuitry in the olfactory bulb that helps detect, decode, and process olfactory information that the brain receives from the external environment.
To understand the underlying neural circuits and mechanisms involved in the detection and discrimination of odors, the Arenkiel team set about finding the targets of GABA and dopamine in sSACs.
First, they found GABA and D1 dopamine receptors, which are present throughout the olfactory bulb and, importantly, on the known and new targets of sSACs, were involved in odor detection.
Using Channelrhodopsin-assisted circuit mapping (CRACM), they found that although sSACs are connected to external tufted cells via both GABA and dopamine receptors, they only release dopamine to granule cells. Furthermore, they found that impaired release of sSAC GABA or dopamine impacts mitral cell firing frequency, which in turn increases the number of glomeruli that respond to a given odor and leads to reduced odor discrimination.
“This study provides crucial mechanistic insights into co-transmission specifically in the context of olfaction and shows how this type of neuromodulation sculpts distinct responses to sensory inputs,” Dr. Arenkiel, who is also a McNair Scholar, said. “It also nicely illustrates how co-transmission allows a single cell type to mount varied responses to the same stimuli by differentially modulating different target neurons. Given that co-transmission is now known to occur in various brain cell types, this will serve as the foundation for further explorations on neuromodulatory effects of multiple neurotransmitters in olfaction as well as in other sensory processes.”
Others involved in the study were Evelyne Tantry, Elizabeth Moss, Mikhail Kochukov, Benjamin Belfort, Joshua Ortiz-Guzman, and Zachary Freyberg. They are affiliated with one or more of the following institutions: the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, Baylor College of Medicine, and the University of Pittsburgh. The study was funded by the National Institutes of Health, McNair Medical Institute at The Robert and Janice McNair Foundation, and the Department of Defense.